-
Looking for HSC notes and resources? Check out our Notes & Resources page
Primary amines (1 Viewer)
- Thread starter =)(=
- Start date
jeez that is a huge atar aim - how are you tracking for thatWould they have 2 H-bonds per molecule cause of the two hydrogens on nitrogen or is it only 1 H bond cause nitrogen only has 1 lone pair?
I always thought it was 2 (1 per hydrogen):
H bonds form between an H (attached to N, O, F --electroneg difference needs to be great enough) and so the H's of an amine can form a hydrogen bond each...?
update just found this diagram if it helps???
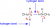
its_ace21
/æɪs/
says the one with a 98 aim and getting 69 in math papersjeez that is a huge atar aim - how are you tracking for that
At the moment its possible just gotta grind rlly hard, loljeez that is a huge atar aim - how are you tracking for that
I always thought it was 2 (1 per hydrogen):
H bonds form between an H (attached to N, O, F --electroneg difference needs to be great enough) and so the H's of an amine can form a hydrogen bond each...?
update just found this diagram if it helps???
View attachment 39640
Ahh interesting so it seems only 1 H bond, cheers
wizzkids
Active Member
- Joined
- Jul 13, 2016
- Messages
- 275
- Gender
- Undisclosed
- HSC
- 1998
This is not a valid question. Perhaps you have forgotten the fundamental nature of hydrogen bonding. Hydrogen bonds only last for between one to ten picoseconds. They are highly transitory and subject to statistical distribution and therefore cannot be counted as such. All we can say is there is a certain probability of an average number of hydrogen bonds per molecule, and this gives rise to increased attractive forces between molecules on average. We know how many charge centres are located on the molecule, but we can't say for sure how many hydrogen bonds a molecule will have at any given instant.
Experiments and calculations done on liquid water showed that although each water molecule is capable of forming four hydrogen bonds, in fact this is highly dependent on time and temperature, and at 25oC water forms about 3.5 hydrogen bonds per molecule.
We can say for sure that primary amines are more strongly attracted than secondary amines, for the same molecular weight, but you can't count the bonds that will occur on any given molecule; this is simply an invalid concept.
Experiments and calculations done on liquid water showed that although each water molecule is capable of forming four hydrogen bonds, in fact this is highly dependent on time and temperature, and at 25oC water forms about 3.5 hydrogen bonds per molecule.
We can say for sure that primary amines are more strongly attracted than secondary amines, for the same molecular weight, but you can't count the bonds that will occur on any given molecule; this is simply an invalid concept.
but... whilst it may be an invalid concept it still makes it much easier to deduce what will be more attracted to what - so what's the issue?This is not a valid question. Perhaps you have forgotten the fundamental nature of hydrogen bonding. Hydrogen bonds only last for between one to ten picoseconds. They are highly transitory and subject to statistical distribution and therefore cannot be counted as such. All we can say is there is a certain probability of an average number of hydrogen bonds per molecule, and this gives rise to increased attractive forces between molecules on average. We know how many charge centres are located on the molecule, but we can't say for sure how many hydrogen bonds a molecule will have at any given instant.
Experiments and calculations done on liquid water showed that although each water molecule is capable of forming four hydrogen bonds, in fact this is highly dependent on time and temperature, and at 25oC water forms about 3.5 hydrogen bonds per molecule.
We can say for sure that primary amines are more strongly attracted than secondary amines, for the same molecular weight, but you can't count the bonds that will occur on any given molecule; this is simply an invalid concept.
wizzkids
Active Member
- Joined
- Jul 13, 2016
- Messages
- 275
- Gender
- Undisclosed
- HSC
- 1998
All hydrogen bonds are not the same. We can assign a bond enthalpy to the hydrogen bonds, and typical enthalpies can range from -1 or -2 kJ/mol up to as much as -25 kJ/mol. You can talk about the potential for more hydrogen bonding between molecules. The difference between amide and carboxylic acid hydrogen bonding revolves around the strength of the hydrogen bonding; differing electronegativities of the oxygen and the nitrogen atoms give rise to different bond enthalpy.
Data on boiling points suggests than amides have stronger hydrogen bonding than carboxylic acids of equivalent molecular weight due to the higher hydrogen bond enthalpy, e.g. ethanamide CH3CONH2 b.p. 221oC versus ethanoic acid CH3COOH b.p. 118oC
Data on boiling points suggests than amides have stronger hydrogen bonding than carboxylic acids of equivalent molecular weight due to the higher hydrogen bond enthalpy, e.g. ethanamide CH3CONH2 b.p. 221oC versus ethanoic acid CH3COOH b.p. 118oC
Last edited:
As the diagram in SadCeliac's post (reproduced below) shows, for an H-bond you need both the hydrogen bond donor (the H atom bound to the very electronegative N, O, or F atom) and the hydrogen bond acceptor (the lone pair on the second very electronegative atom). So, a primary amine like methanamine can, with two donors but only one receptor, can only average one H-bond per molecule. There will be some molecules with both H's involved in an H-bond, and some with none, but any average of one per molecule is reasonable.Would they have 2 H-bonds per molecule cause of the two hydrogens on nitrogen or is it only 1 H bond cause nitrogen only has 1 lone pair?
For this reason, an alcohol and an amine will have similar amounts of H-bonding. The alcohol will typically have the higher BP as the H-bonds in the amine are weaker (O is more electronegative than N) and because of steric factors (it is easier to get the one donor H of an alcohol near to one of the two acceptor lone pairs than it is to get one of the two donor H's of a primary amine near the one acceptor lone pair.
In the common year 11 question showing the BP trends of the group 4, 5, 6, and 7 hydrides against molar mass (where HF, H2O, and NH3 all have much higher BPs than any of the other species due to H-bonding), the BP(H2O) = 100 oC is greater than both BP(HF) = 20 oC and BP(NH3) = -33 oC because only water has 2 donors and 2 acceptors and is thus capable of double the H-bonding of HF (1 donor, 3 acceptors) or NH3 (3 donors, 1 acceptor). The graph is available on the Doc Brown Chemistry notes website at https: //docbrown.info/page07/equilibria8f.htm .
I've been taught that amides have higher BP than carboxylic acids of similar molar mass.All hydrogen bonds are not the same. We can assign a bond enthalpy to the hydrogen bonds, and typical enthalpies can range from -1 or -2 kJ/mol up to as much as -25 kJ/mol. You can talk about the potential for more hydrogen bonding between molecules. The difference between amide and carboxylic acid hydrogen bonding revolves around the strength of the hydrogen bonding; differing electronegativities of the oxygen and the nitrogen atoms give rise to different bond enthalpy.
Carboxylic acids have much higher boiling points than amides of equivalent molecular weight due to the higher hydrogen bond enthalpy.
This is certainly true in the example in the Caringbah 2023 Chemistry Trial that was recently uploaded, question 34, which gives the following data:
- Propanoic acid, CH3CH2COOH, has molar mass 74.08 g mol-1 and BP = 141.2 oC.
- Propanamide, CH3CH2CONH2, has molar mass 73.09 g mol-1 and BP = 213 oC.
Similar data for the two carbon compounds:
- Acetic acid, CH3COOH, has molar mass 60.052 g mol-1 and BP = 118 oC.
- Acetamide, CH3CH2CONH2, has molar mass 59.07 g mol-1 and BP = 222 oC.
- BP(formamide, HCONH2) = 210 o > BP(formic acid, HCOOH) = 100.8 oC.
