krypticlemonjuice
Member
- Joined
- Jun 24, 2023
- Messages
- 92
- Gender
- Male
- HSC
- 2023
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
Hmmm i see what you’re saying. what does the angle have to do in this question. Which formula links greatest wavelength and energy with the angle ??Four different wavelengths of light are being released, and as such four different interference patterns are visible. By the formula for double slit, we can basically say that m=1 for the patterns around Q (since they are the first from the middle), and therefore Q is the one where θ is greatest, so sinθ is greatest and hence we’re looking for the energy transition which releases the greatest wavelength. You can test this for all of them with the formula for energy transitions but basically 1/λ is lowest for the closest transition, which is 3->2, and hence λ is greatest for 3->2, so the answer must be A.
Hmmm i see what you’re saying. what does the angle have to do in this question. Which formula links greatest wavelength and energy with the angle ??
Also is this a general result. Based on the formula, will the transition from 3 —> 2 always be the result with the greatest energy transition.
If a longer wavelength is released, doesn’t that mean less energy is released as frequency is lower ?
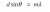
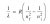
Ahh I see I see, thank you for your explanation!View attachment 38824
View attachment 38823
The energy transition that releases the greatest wavelength isn’t the one that releases the most energy, it’s actually the opposite.
All that this result means is that out of the Balmer series (transitions from any number to 2), 3->2 releases the longest wavelength.

