Michaelmoo
cbff...
- Joined
- Sep 23, 2008
- Messages
- 590
- Gender
- Male
- HSC
- 2009
Just wondering. Could anyone please explain to me why Sodium hydrogen sulfate is an acidic salt (really acidic in fact).
Thats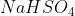
Thats
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
Sure it's really acidic?Just wondering. Could anyone please explain to me why Sodium hydrogen sulfate is an acidic salt (really acidic in fact).
Thats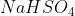
Yer man. Bout 1.4-1.9pH for a 1M apparently.Sure it's really acidic?
HSO4- is a weak acid.
Ok. Yes makes sense. But I still don't understand. That equation doesn't take into acount:
You've got it.Ok. Yes makes sense. But I still don't understand. That equation doesn't take into acount:
- The system is in equilibrium, the reaction does not go to completion
- The second ionisation of sulfuric acid is essentially weak... Where are all these hydrogen ions coming from?
Ahh k. Makes sense. Just thought it was quite unusual for an ion in equillibrium to generate a relatively acidic solution.You've got it.
it's exactly the second ionisation of sulfuric acid. It's a weak acid but it is quite a strong weak acid (if that makes sense).
It does not completely ionise but quite a lot of it actually does. And remember that 1 change in pH is equivalent to 10 fold change in [H+]. Thus pH of 1.9 for 1M means that it's about 10 times weaker than HCl.
Hehe thanks u 2No worries, good luck for your MX1 tomorrow (that is, if you are gonna look at this post tonight lol)

