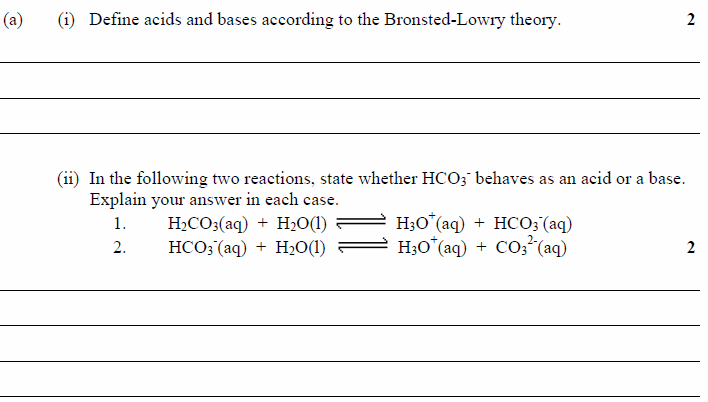
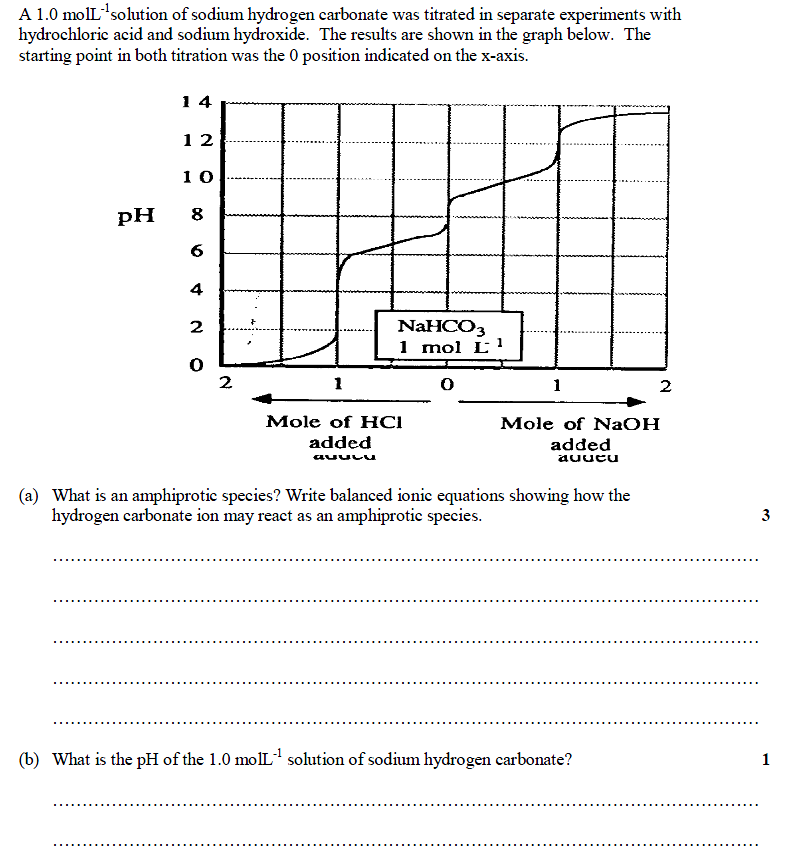
Re: HSC 2012 Chemistry Marathon
Hi guys, this is a question from the 2009 HSC -
Question 20:
Calculate the mass of ethanol that must be burnt to increase the temp of 210g of water by 65 degrees celsius if exactly half of teh heat released by this combustion is lost to its surroundings.
Heat of combustion = 1367 kj/mol/L
many thanks in advance
Hi guys, this is a question from the 2009 HSC -
Question 20:
Calculate the mass of ethanol that must be burnt to increase the temp of 210g of water by 65 degrees celsius if exactly half of teh heat released by this combustion is lost to its surroundings.
Heat of combustion = 1367 kj/mol/L
many thanks in advance