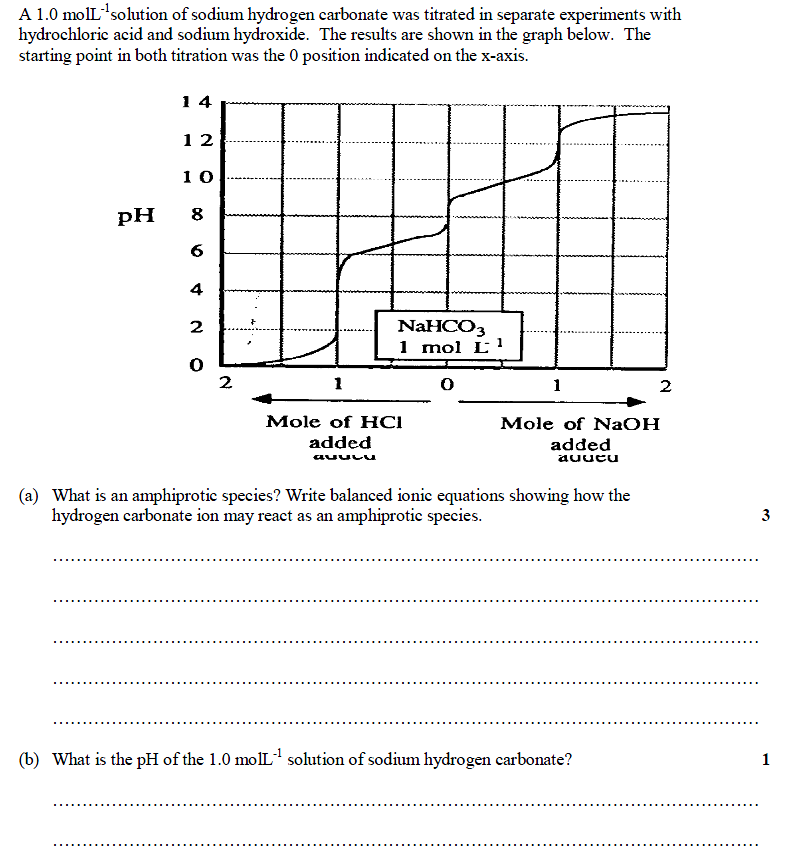
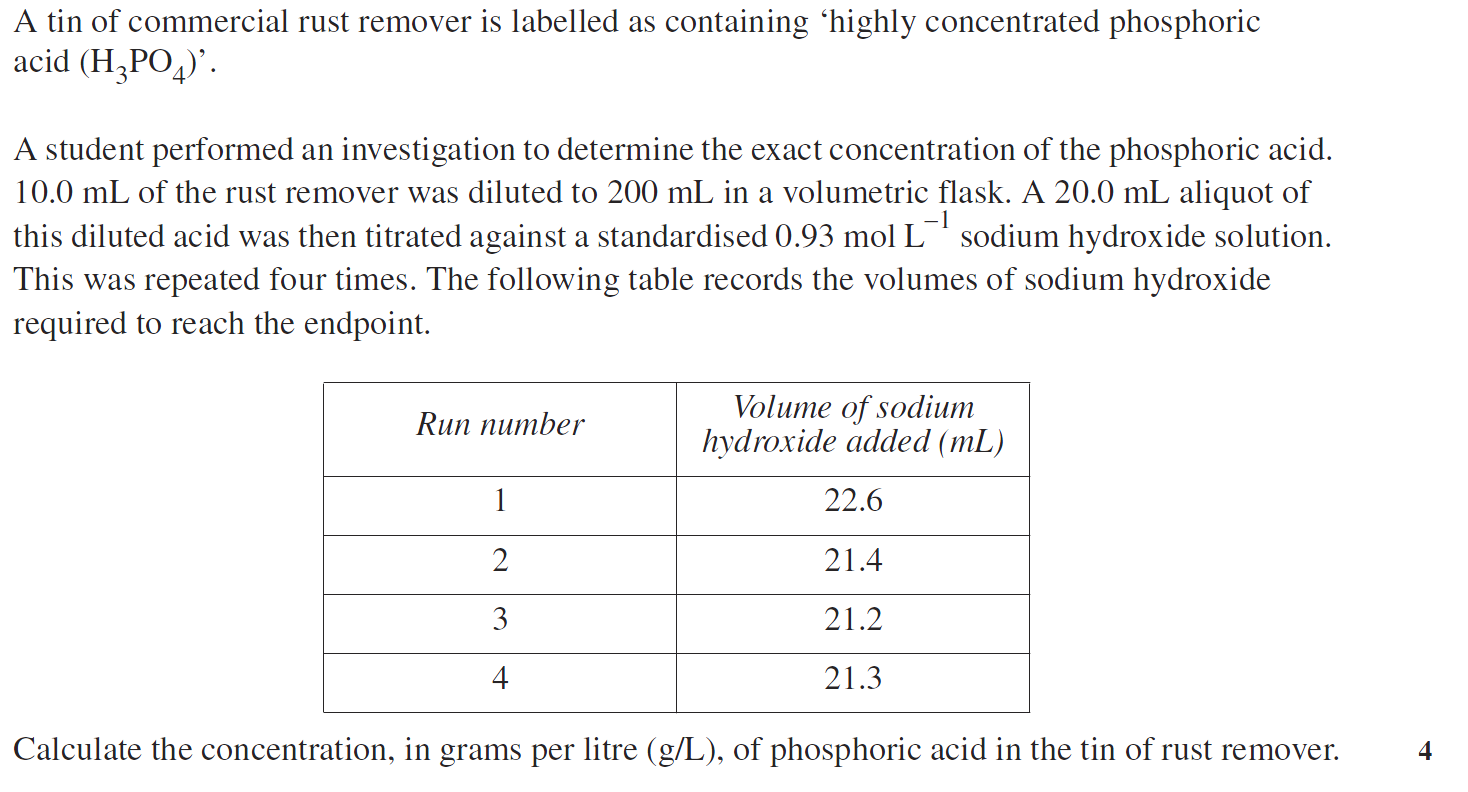
hscwav2012
Member
Re: HSC 2012 Chemistry Marathon
This is from Independent 09 Trial Exam
Multiple Choice Q3:
3. The molar heat of combustion of ethanol is 1367kJ/mol
What mass of ethanol is required to heat 1.0 moles of water by 10 degrees.
a - 136.7g
b - 46.0g
c - 25.3g
d - 0.025g
This is from Independent 09 Trial Exam
Multiple Choice Q3:
3. The molar heat of combustion of ethanol is 1367kJ/mol
What mass of ethanol is required to heat 1.0 moles of water by 10 degrees.
a - 136.7g
b - 46.0g
c - 25.3g
d - 0.025g