Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
Anaerobic Respiration (1 Viewer)
- Thread starter axwe7
- Start date
axwe7
Member
- Joined
- Aug 4, 2015
- Messages
- 183
- Gender
- Undisclosed
- HSC
- N/A
Wouldn't 2 represent the number of molecules not moles?You do Chemistry don't you? So doesn't the "2" equal the number of moles and it won't change the molecules name?
I get that, but what I'm confused about, is that if you expand the Lactic Acid, it would be 2C3 + 2H6 + 2O3 correct?
Btw, I'm starting preliminary on Thursday...
Queenroot
I complete the Squar3
It represents the number of moles.
Queenroot
I complete the Squar3
And no it's not glucose
2C3H6O3 simply means 2 molecules (or moles not sure) of lactic acid. Even though there equal number of atoms of carbon hydrogen and oxygen atoms, they are not bonded in such a way that they turn out to be glucose, get it? It like saying 2 molecules of hydroxide (OH) is actually hydrogen peroxide (H2O2), Which is definitely not true
Last edited:
axwe7
Member
- Joined
- Aug 4, 2015
- Messages
- 183
- Gender
- Undisclosed
- HSC
- N/A
Thanks.... I've understood it now2C3H6O3 simply means 2 molecules (or moles not sure) of lactic acid. Even though there equal number of atoms of carbon hydrogen and oxygen atoms, they are not bonded in such a way that they turn out to be glucose, get it? It like saying 2 molecules of hydroxide (OH) is actually hydrogen peroxide (H2O2), Which is definitely not true
axwe7
Member
- Joined
- Aug 4, 2015
- Messages
- 183
- Gender
- Undisclosed
- HSC
- N/A
Gotcha <3Glucose is C6H12O6
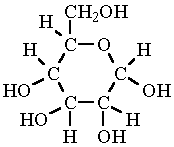
lactic acid is C3H6O3
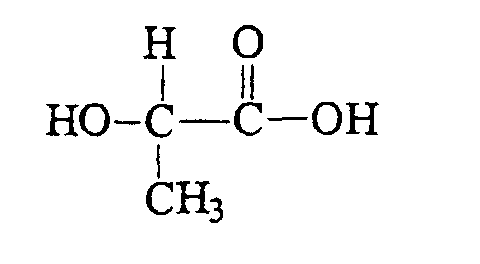
Look at the difference in structure
Thanks

