ichila101
Split arrowhead
And this is all due to electronegativities of each element I'm guessing? So for example if they had asked in an exam whether Silicon Dioxide was a Covalent compound or not and the only info I knew at the time was that Covalent compounds occur between non-metals (plus the fact the exam did not provide an electronegativity periodic table), would I be wrong if I said it wasn't a Covalent compound? How would you really know whether something is a Covalent Compound or not if they don't show the electrons sharing between atoms or they don't show the electronegativity table (since you said it can occur between metalloids and metals)?
Actually it has more to do with the valence shells of the elements (not sure if you have learnt about valence shells) but they are essentially the last shell of an element and because elements that do not 8 electrons in their last shells they are reactive and want to gain or lose electrons in some cases they cant (e.g. with covalent bonds) so they share them which allows them to form covalent bondsAnd this is all due to electronegativities of each element I'm guessing?
Yes you would be wrong if you said that it wasnt a covalent compound because it is a covalent compound. Whether or not you had that information I am sure that you would get that question wrong if you had that as your answerSo for example if they had asked in an exam whether Silicon Dioxide was a Covalent compound or not and the only info I knew at the time was that Covalent compounds occur between non-metals (plus the fact the exam did not provide an electronegativity periodic table), would I be wrong if I said it wasn't a Covalent compound?
Well I am sure they wont overcomplicate questions and ask that, if they do I am pretty sure that would all work out if you just memorise your notes (which i really dont like especially in science subjects) so there are some ways to do this although for this question it should not be too hard since there are very few mentioned covalent network substances.How would you really know whether something is a Covalent Compound or not if they don't show the electrons sharing between atoms or they don't show the electronegativity table (since you said it can occur between metalloids and metals)?
Generally that kind of question would be a lot harder to solve using valencies for a yr 9/10 student so you would have to simply use the process of elimination.
For example for Silicon dioxide, it quite obviously cannot be a metallic compound since, as the name suggests, metallic compounds would only include metals
Also it cannot be a ionic compound because neither the silicon element nor the oxygen molecule are completely giving away or gaining electrons from each other.
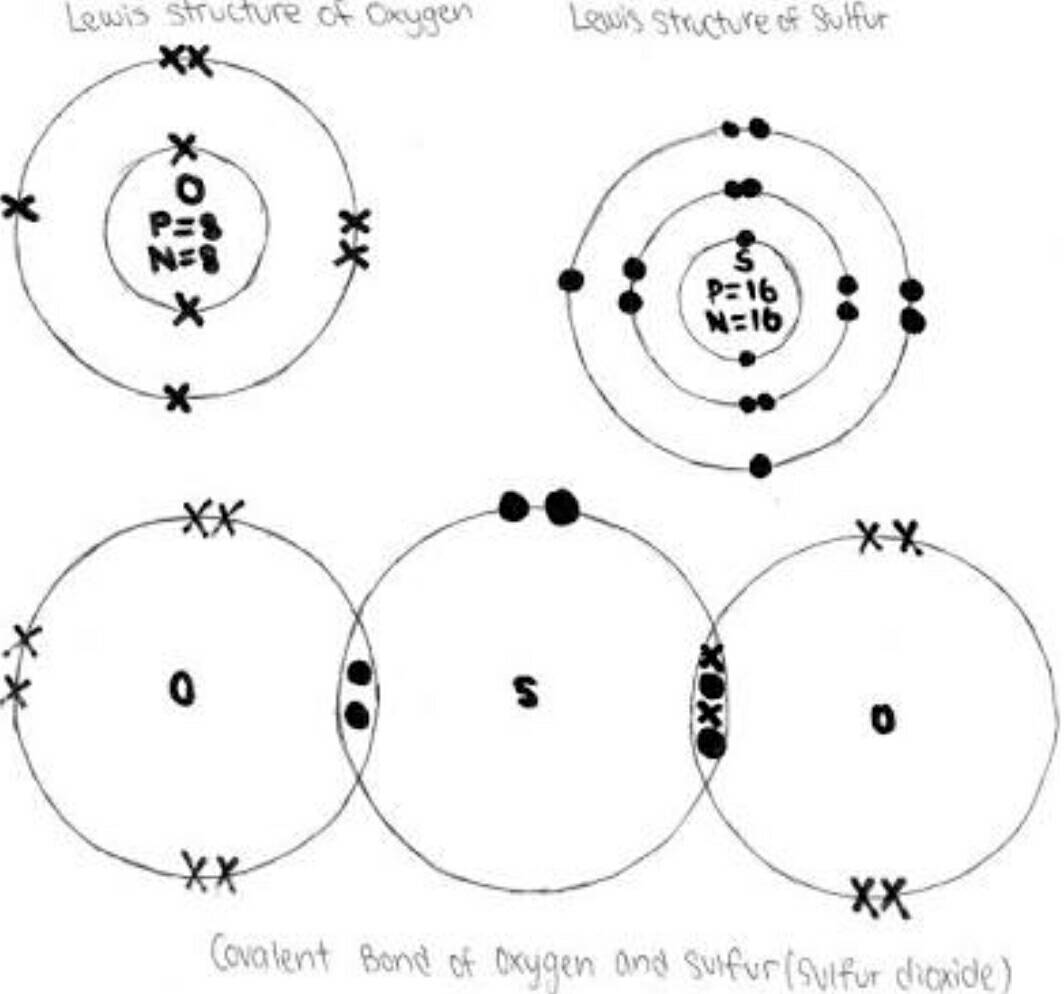
If you were to explain it in year 12 (you probably wouldnt be asked this type of question though) you could simply describe the bonds that would occur between the elements as shown below
On the right between the sulfur and oxygen element there is clearly a covalent bond; sharing of electrons and on the left side of the sulfur and left oxygen there is what is known as a 'coordinate covalent bond' something that is learnt in year 12 which you dont need to worry about but I just told you to understand that in terms of structure it would be made up of covalent bonds (btw just realised that you may not have learnt lewis dot diagrams and most of this stuff is year 11, tell me if you havent learnt lewis diagrams if you want me to explain but if you already understand them then its all good)
Sent from my SM-G900I using Tapatalk
