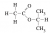
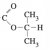
I got this, would this also be correct? Since it matches with the graphs
View attachment 32712
No, it would not, for two reasons, one minor, one major.
The minor one is the C atom adjacent to the C of the carboxyl group is missing an H atom.
The major one is that the signal for that methine (CH) carbon, which is split into a septet, occurs at a chemical shift of something like 5. This is way too far downfield to be the effect of just an adjacent carbonyl or carboxyl, it points to a direct bond to an electronegative atom like an O. Similarly, the singlet methyl group that occurs at around 2.1 ppm, which is consistent with an adjacent carbonyl or carboxyl, would have a chemical shift of perhaps 4.5 ppm or more if it were bonded to an O atom as you have drawn.
1H NMR gives four varieties of information:
- number of signals = number of environments
- chemical shift of each signal tells about the neighbouring environment in terms of electron density and shielding / deshielding
- splitting tells about the presence of NMR active nuclei on the neighbouring atoms; for HSC, we look only at splitting by neighbouring H's
- intensity / integration tells about the ratio of 1H atoms in the environments, usually giving the number of H atoms.
You need to consider each. The difference between methyl 2-methylpropanoate (your structure) and 2-propyl acetate (the correct answer) is in the second point, the chemical shifts. Both compounds have 3
1H environments, in a 1:3:6 ratio, and with the splitting being (in the order of the intensity ratio) septet - singlet - doublet, so they are indistinguishable from the other three varieties of information, but on chemical shifts, they are clearly different:
methyl 2-methylpropanoate has a singlet (3H, d = 4.5 ppm), a septet (1H, d = 2.1 ppm), and a doublet (6H, d = 0.9 ppm).
2-propyl acetate has a septet (1H, d = 5 ppm), a singlet (3H, d = 2 ppm), and a doublet (6H, d = 1.2 ppm).
Note: Chemical shifts are approximations.