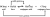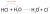Question in title
I've always just assumed that the lower pKa is the stronger the acid, and the higher pKb is the stronger the base - similar to pH. But I think there's a better way to explain this in a exam - could yall please help??
Thanksss
I've always just assumed that the lower pKa is the stronger the acid, and the higher pKb is the stronger the base - similar to pH. But I think there's a better way to explain this in a exam - could yall please help??
Thanksss