I agree mostly with the account given by
@carrotsss which is very well done. A good description of dynamic equilibrium.
However, there is one feature missing - the transport problem. The radioactive Pb
2+ ions will leach out of the
surface of the solid and be replaced with non-radioactive Pb
2+ ions on the surface of the solid. On this part, we are in agreement. However the interior of the solid will be unchanged. There will be a slow build-up of radioactivity in the solution, but this build-up will slow down due to the transport problem in the solid. In order for radioactive Pb
2+ ions to continue to reach the surface between the solid and the liquid, they have to diffuse through the solid phase to reach the surface, which is an extremely slow process compared to diffusion in the liquid phase. Hence we will get a negative exponential curve, and the distribution of radioactive Pb
2+ ions will look something like this:
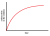
You will encounter this phenomenon in
Module 8 under Chemical Synthesis and Design - reaction conditions. You might like to think about what might be done by changing the reaction conditions to speed up the process of reaching equilibrium.