-
Looking for HSC notes and resources? Check out our Notes & Resources page
Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
alpha-glucose beta-glucose (1 Viewer)
- Thread starter jackc91
- Start date
study-freak
Bored of
- Joined
- Feb 8, 2008
- Messages
- 1,128
- Gender
- Male
- HSC
- 2009
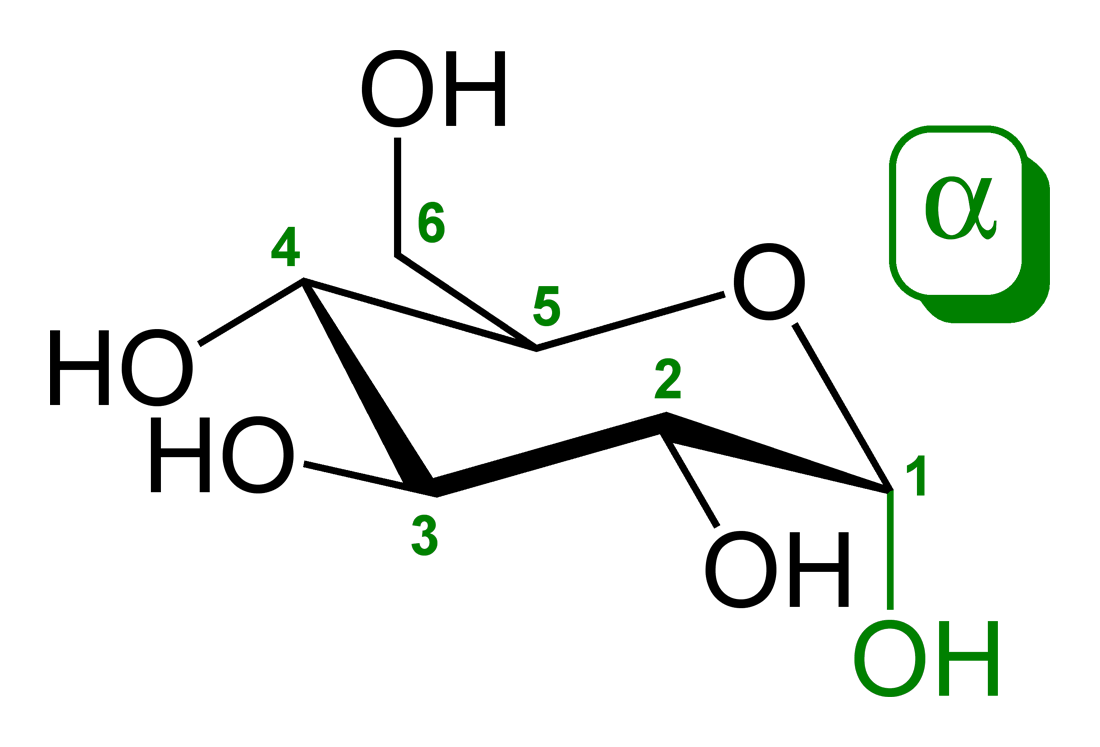
Alpha and beta glucose are both glucoses yet they have a slightly different structure. The difference in structure is summarised by "alpha is down, beta is up"-for hydroxyl groups at one end of the ring structure. Basically the only difference is the position of a hydroxyl group.
You might need to see a simplified diagram of both glucoses to understand what I'm talking about but cbb scanning them and posting- sorry xD.
google should give u the pictures (or some other replys)
You might need to see a simplified diagram of both glucoses to understand what I'm talking about but cbb scanning them and posting- sorry xD.
google should give u the pictures (or some other replys)
Last edited:
Pwnage101
Moderator
isn't it 'beta is up, alpha is down' ?study-freak said:Alpha and beta glucose are both glucoses yet they have a slightly different structure. The difference in structure is summarised by "alpha is up, beta is down"-for hydroxyl groups at one end of the ring structure. Basically the only difference is the position of a hydroxyl group.
You might need to see a simplified diagram of both glucoses to understand what I'm talking about but cbb scanning them and posting- sorry xD.
google should give u the pictures (or some other replys)
EDI: It all depends on how you've drawn it - if the CH2OH attached to C-5 is 'up and the OH on C-1 is also up, then it is beta glucose.
see wikipedia: "When D-glucose is drawn as a Haworth projection or in the standard chain conformation, the designation α means that the hydroxyl group attached to C-1 is positioned trans to the -CH2OH group at C-5, while β means it is cis. An inaccurate but superficially attractive alternative method of distinguishing α from β is by observing whether the C-1 hydroxyl is below or above the plane of the ring; this may fail if the glucose ring is drawn upside down or in an alternative chair conformation. "

Last edited:
Timothy.Siu
Prophet 9
beta is for cellulose i think lol
and the funny group alternates up and down or something
and the funny group alternates up and down or something
study-freak
Bored of
- Joined
- Feb 8, 2008
- Messages
- 1,128
- Gender
- Male
- HSC
- 2009
I'm sorry, i have made a srs mistake.Pwnage101 said:isn't it 'beta is up, alpha is down' ?
edited the previous post
Last edited:
Pwnage101
Moderator
no worriesstudy-freak said:I'm sorry, i have made a srs mistake.
edited the previous post
gurmies
Drover
Both alpha and beta alternate in terms of polymer structure....beta for cellulose, alpha for starch polymers
Small but useless fact: because the OH group alternate in the case of beta glucose, it causes the polarity of polymer to cancel out, resulting in the non-water soluability of cellulose. For the polymer of alpha glucose, the OH is aligned towards the same side of the polymer chain, making it much more water soluable.
Also, alpha and beta glucose equilibrate in water.
Also, alpha and beta glucose equilibrate in water.
Pwnage101
Moderator
interesting, yeh my teacher taught me how in water they equilibrate
