While I agree with
@jazz519's reasoning, I think the conclusion drawn in an answer regarding spontaneity depends on perspective.
If you take the view that a reaction is spontaneous if
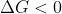
and non-spontaneous if
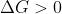
, then you might answer that:
- as temperature increases, the value of
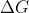 will increase as
will increase as 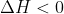 and
and 
- as
 , there must be a critical temperature
, there must be a critical temperature 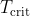 at which
at which 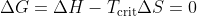
- the reaction will be spontaneous so long as
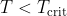 , and will be non-spontaneous when
, and will be non-spontaneous when 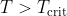
An example of this perspective is to consider the freezing of water, H
2O(l) ---> H
2O(s), which is exothermic (
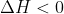
) and entropically disfavoured (

), like the above example. For pure water under standard conditions, freezing is spontaneous at any temperature below 0
oC and non-spontaneous (i.e. the reverse process, melting, is spontaneous) at any temperature above 0
oC. Is it really meaningful to say that water freezes "more spontaneously" at (say) -5
oC than at -4
oC, or that spontaneity is greater?
Certainly, there are books / teachers / chemists who use language like "greater spontaneity" to mean that an already-negative
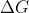
is decreasing,. Describing this as a
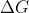
becoming "more negative" is linguistically debatable, as is the view of spontaneity as a relative concept that can be present in greater or lesser amounts, rather than as an absolute that is either present or absent under a given set of conditions. I favour the latter perspective and so would not say that " the deltaG is becoming more positive" when I mean that
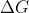
is negative but increasing towards zero as temperature increases because I don't see why -5 kJ mol
-1 is "more positive" than -10 kJ mol
-1.
I would answer:
As temperature increases, Gibbs Free Energy increases but the reaction remains spontaneous until the temperature passes the threshold at which
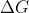
becomes positive, where the reaction becomes non-spontaneous. We are told that the reaction is exothermic (
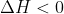
) and that the reaction proceeds (thus,
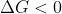
), but the reaction is entropically disfavoured (

) as order increases when two species combine to form one in the same state. Since

, increasing temperatures will increase the value of the positive term (
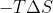
) and so cause
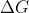
to increase from its initial negative value, ultimately to become positive at some critical temperature, at which point the reaction ceases to be spontaneous.