Right, sorry. It is a 20.00mL aliquot.I feel like there is some info missing in this question. How much of that hcl excess solution is titrated against the naoh. Otherwise if it’s the whole thing you are going to get a crazy different number to the manufacturers one
-
Coming soon...BoS Trial exams Watch this space!
Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
Chemistry New Syllabus Advice / Sample Answers by an Experienced HSC Tutor (1 Viewer)
- Thread starter jazz519
- Start date
In back titration, the reason people find it hard is because the way they approach these questions is to try do everything in one go or an illogical process or they have poor layout for their answer. Being specific in terms of what moles you are calculating such as excess or initial or reacted here is really importantBack titration question:
20.00mL of cloudly ammonia was pipetted into a 250mL volumetric flask. 100mL of 0.5866M HCl in excess was added. The volume was made up to 250mL with distilled water. A burette was filled with 0.1194M NaOH (standardised) solution. The HCl and ammonia solution was titrated with the NaOH, with the mean titre being 22.75mL.
The manufacturer of the cloudy ammonia claims that the detergent contains 45.2gL-1 ammonia as ammonium hydroxide (NH4OH).
a) Calculate the concentration in gL-1 of NH4OH in the cloudly ammonia sample
b) Provide possible explainations for differences between the calculated results and the manufacturers claims
Thanks Jazz
To do back titration you should do a backwards approach. Meaning start analysing the question from the bottom first and then move up towards the top
Following this approach first summarise the sentence: The HCl and ammonia solution was titrated with the NaOH, with the mean titre being 22.75mL.
NaOH(aq) + HCl(aq) --> NaCl(aq) + H2O(l)
V(NaOH) = 22.75 mL
Then summarise the sentence before it:
C(NaOH) = 0.1194 M
n(NaOH) = CV = (0.1194)(22.75/1000) = 2.71635 x 10^-3 moles
This is the NaOH reacted with the excess HCl, so therefore:
n(HCl) excess in 20.00 mL aliquot = n(NaOH) = 2.71635 x 10^-3 moles
Then continue with that approach:
Because only 20.00 mL aliquots were taken, this means that we need to change it to 250 mL. To do this just think about the ratio:
n(HCl) excess is 1.00 mL = (2.71635 x 10^-3)/20.00 = 1.358175 x 10^-4 moles
so n(HCl) excess in 250.0 mL = (1.358175 x 10^-4)x250.0 = 0.033954375 moles
Other reaction occurring is:
NH4OH(aq) + HCl(aq) --> NH4Cl(aq) + H2O(l)
n(HCl) initial = CV = (0.5866)(0.100) = 0.05866 moles
n(HCl) reacted with NH4OH = n(HCl) initial - n(HCl) excess
n(HCl) reacted with NH4OH = 0.05866 - 0.033954375
n(HCl) reacted with NH4OH = 0.024705625 moles
n(NH4OH) = n(HCl) reacted with NH4OH
n(NH4OH) = 0.024705625 moles
m(NH4OH) = n x MM = (0.024705625)(14.01+5x1.008+16) = 0.8659321563 g
This is NH4OH in 20.00 mL of the cloudy ammonia.
Therefore, g/L conc = (0.8659321563) / (20.00 x 10^-3) = 43.2966... g/L = 43.3 g/L
Thanks so much for the fast reply! I was having trouble with how the dilution comes into the question but you cleared it up nicely (and also the structure of back titration problems)In back titration, the reason people find it hard is because the way they approach these questions is to try do everything in one go or an illogical process or they have poor layout for their answer. Being specific in terms of what moles you are calculating such as excess or initial or reacted here is really important
To do back titration you should do a backwards approach. Meaning start analysing the question from the bottom first and then move up towards the top
Following this approach first summarise the sentence: The HCl and ammonia solution was titrated with the NaOH, with the mean titre being 22.75mL.
NaOH(aq) + HCl(aq) --> NaCl(aq) + H2O(l)
V(NaOH) = 22.75 mL
Then summarise the sentence before it:
C(NaOH) = 0.1194 M
n(NaOH) = CV = (0.1194)(22.75/1000) = 2.71635 x 10^-3 moles
This is the NaOH reacted with the excess HCl, so therefore:
n(HCl) excess in 20.00 mL aliquot = n(NaOH) = 2.71635 x 10^-3 moles
Then continue with that approach:
Because only 20.00 mL aliquots were taken, this means that we need to change it to 250 mL. To do this just think about the ratio:
n(HCl) excess is 1.00 mL = (2.71635 x 10^-3)/20.00 = 1.358175 x 10^-4 moles
so n(HCl) excess in 250.0 mL = (1.358175 x 10^-4)x250.0 = 0.033954375 moles
Other reaction occurring is:
NH4OH(aq) + HCl(aq) --> NH4Cl(aq) + H2O(l)
n(HCl) initial = CV = (0.5866)(0.100) = 0.05866 moles
n(HCl) reacted with NH4OH = n(HCl) initial - n(HCl) excess
n(HCl) reacted with NH4OH = 0.05866 - 0.033954375
n(HCl) reacted with NH4OH = 0.024705625 moles
n(NH4OH) = n(HCl) reacted with NH4OH
n(NH4OH) = 0.024705625 moles
m(NH4OH) = n x MM = (0.024705625)(14.01+5x1.008+16) = 0.8659321563 g
This is NH4OH in 20.00 mL of the cloudy ammonia.
Therefore, g/L conc = (0.8659321563) / (20.00 x 10^-3) = 43.2966... g/L = 43.3 g/L
- Common Error of students: NOT drawing all bonds in an organic molecule
A common error by students in Chemistry exams relating to questions in Module 7: Organic Chemistry, is not showing all the bonds in a structure or doing things like not drawing hydrogens in
Directly from the HSC markers comments in the 2015 HSC Chemistry Paper: "
Candidates need to improve in these areas: ....
An example of an answer that could be penalised is shown below:
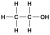
Look very careful at the OH on the right hand part of the molecule. The bond between the oxygen and hydrogen has been omitted
An answer that shows that bond is shown below:
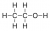
Other examples of this include, not drawing the bonds in a CH3. The CH3 on the diagram in the left diagram should be expanded as shown in the diagram on the right hand image:
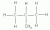
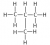
A common error by students in Chemistry exams relating to questions in Module 7: Organic Chemistry, is not showing all the bonds in a structure or doing things like not drawing hydrogens in
Directly from the HSC markers comments in the 2015 HSC Chemistry Paper: "
Candidates need to improve in these areas: ....
- drawing full structural formulae showing all bonds, including the O-H bond (Q26a)"
An example of an answer that could be penalised is shown below:

Look very careful at the OH on the right hand part of the molecule. The bond between the oxygen and hydrogen has been omitted
An answer that shows that bond is shown below:

Other examples of this include, not drawing the bonds in a CH3. The CH3 on the diagram in the left diagram should be expanded as shown in the diagram on the right hand image:


Sometimes when you react an acid and base the salt may not be neutral. It can be acidic, basic or neutralA bit confused on this question
Which is an acidic salt?
(A) CH3COONa
(B) NaHCO3
(C) NH4Cl
(D) NaCl
You first have to know what makes an acid/base strong or weak
if you know that then it’s just pick whichever thing overpowers the other one
Strong acid + strong base = neutral (nothing overpowers)
Strong acid + weak base = acidic (acid has overpowered)
weak acid + strong base = basic (base has overpowered)
So all these salts are made from neutralisation reactions.
Ch3cooNa comes from ch3cooh + naoh meaning acetic acid is weak acid, sodium hydroxide is strong base so the salt is basic
nahco3 comes from h2co3 and naoh. As carbonic acid is weak acid and sodium hydroxide is strong base. The salt is basic
Nh4cl comes from nh3 and hcl. Nh3 is weak base, hcl is strong acid. So the salt is acidic. So this is your answer C
nacl comes from naoh and hcl. Naoh is strong base, hcl is strong acid. So the salt is neutral
Carl10101
Member
- Joined
- Aug 29, 2019
- Messages
- 40
- Gender
- Male
- HSC
- 2020
Ah yes I get it now thanks so muchSometimes when you react an acid and base the salt may not be neutral. It can be acidic, basic or neutral
You first have to know what makes an acid/base strong or weak
if you know that then it’s just pick whichever thing overpowers the other one
Strong acid + strong base = neutral (nothing overpowers)
Strong acid + weak base = acidic (acid has overpowered)
weak acid + strong base = basic (base has overpowered)
So all these salts are made from neutralisation reactions.
Ch3cooNa comes from ch3cooh + naoh meaning acetic acid is weak acid, sodium hydroxide is strong base so the salt is basic
nahco3 comes from h2co3 and naoh. As carbonic acid is weak acid and sodium hydroxide is strong base. The salt is basic
Nh4cl comes from nh3 and hcl. Nh3 is weak base, hcl is strong acid. So the salt is acidic. So this is your answer C
nacl comes from naoh and hcl. Naoh is strong base, hcl is strong acid. So the salt is neutral
I'd add chem equations to show that NH4Cl can act as an acidSometimes when you react an acid and base the salt may not be neutral. It can be acidic, basic or neutral
You first have to know what makes an acid/base strong or weak
if you know that then it’s just pick whichever thing overpowers the other one
Strong acid + strong base = neutral (nothing overpowers)
Strong acid + weak base = acidic (acid has overpowered)
weak acid + strong base = basic (base has overpowered)
So all these salts are made from neutralisation reactions.
Ch3cooNa comes from ch3cooh + naoh meaning acetic acid is weak acid, sodium hydroxide is strong base so the salt is basic
nahco3 comes from h2co3 and naoh. As carbonic acid is weak acid and sodium hydroxide is strong base. The salt is basic
Nh4cl comes from nh3 and hcl. Nh3 is weak base, hcl is strong acid. So the salt is acidic. So this is your answer C
nacl comes from naoh and hcl. Naoh is strong base, hcl is strong acid. So the salt is neutral
ignore Cl- as it's a spectator ion.
NH4+ + H2O <--> H3O+ + NH3
production of H3O+ therefore acidic
Yep for short answer you need that but multiple choice just faster to do what I said aboveI'd add chem equations to show that NH4Cl can act as an acid
ignore Cl- as it's a spectator ion.
NH4+ + H2O <--> H3O+ + NH3
production of H3O+ therefore acidic
- Common Calculation Errors - Don't Make These Mistakes & Save Valuable Easy Marks:
Whenever I mark calculation questions of students it always strikes me at almost every single student makes these careless mistakes and it can cost you in the exam, where you need to minimise the amount of marks you lose to silly errors.
Each of these errors can cost you a mark on a question
Common errors in calculation questions include:
- Not putting states on the chemical equation
- Not balancing the chemical equation
- Not writing a formula first before subbing in. You should always follow this approach:
Write formula --> substitute --> evaluate in calculator
- Leaving out units for numbers. It is NOT good enough to just put units on the final answer, you need to put units throughout the answer at every number you have
Here is an example of how your calculation should be structured:

Pay close attention to how every step in the working is clearly set out with formulas written before substituting and every number has a unit (not just the final answer). Also the omission of the outlier was mentioned
Following a strict structure when calculating things will help you better understand the formulas and also may help you have an edge in harder questions, where it is more than just substituting numbers.
Whenever I mark calculation questions of students it always strikes me at almost every single student makes these careless mistakes and it can cost you in the exam, where you need to minimise the amount of marks you lose to silly errors.
Each of these errors can cost you a mark on a question
Common errors in calculation questions include:
- Not putting states on the chemical equation
- Not balancing the chemical equation
- Not writing a formula first before subbing in. You should always follow this approach:
Write formula --> substitute --> evaluate in calculator
- Leaving out units for numbers. It is NOT good enough to just put units on the final answer, you need to put units throughout the answer at every number you have
Here is an example of how your calculation should be structured:

Pay close attention to how every step in the working is clearly set out with formulas written before substituting and every number has a unit (not just the final answer). Also the omission of the outlier was mentioned
Following a strict structure when calculating things will help you better understand the formulas and also may help you have an edge in harder questions, where it is more than just substituting numbers.
This isn't a full working but you should have these numbers:I know how to work this out but I wanted to check my answer is correct:
10.0 mL of a 0.010 molL-1 magnesium hydroxide solution is added to 15.0 mL of 0.020 molL-1 nitric acid solution.
What is the pOH of the final solution?
Mg(OH)2(aq) + 2HNO3(aq) --> Mg(NO3)2(aq) + 2H2O(l)
n(Mg(OH)2) = 0.0001 moles
n(HNO3) = 0.0003 moles
Mg(OH)2 is the limiting reagent, HNO3 is excess
n(HNO3) excess = 0.0003 - 0.0002 = 0.0001 moles
n(H+) = n(HNO3) = 0.0001 moles
c(H+) = n/v = (0.0001)/(25.0 x 10^-3) = 0.004 M
pH = -log(0.004) = 2.3979
pOH = 14 - pH = 11.60
- How to answer Le Chatelier's Principle (LCP) questions (common mistakes):
This is one of the most important question types for the HSC and you can be sure to expect a question on this topic in a variety of different forms:
- Multiple choice questions
- 2-3 marker short answer
- 5-8 marker long response questions
A lot of students know how the principle works and the basics of predicting a left or right shift, but students answers are often lacking in depth when they write out things.
It's not just about getting the correct equilibrium shift, but being able to justify why and linking this to other physical properties such as colours and solubility.
Here are some common mistakes students make when answering these questions:
- Not defining Le Chatelier's Principle in their answer
- Not fully justifying their choice on why the equilibrium shifted left or right
- Forgetting to discuss the changes in concentration caused by the shift in equilibrium
- Forgetting to link the shift to change in colour when the question has specified compounds have a certain colour
Each of these can cost you a mark on an area that if you get in an exam can often be easy guaranteed marks
Here is an Exemplar Answer to this question showing how your answer should be constructed:

Pay attention to:
- Le Chatelier's principle was defined
- The equilibrium shift was justified (and not just simply plainly written as shifting left or right --> you must say why)
- Link to reaction rate
- Comment on the effect on concentrations
- Linked to colours provided in the question
This is one of the most important question types for the HSC and you can be sure to expect a question on this topic in a variety of different forms:
- Multiple choice questions
- 2-3 marker short answer
- 5-8 marker long response questions
A lot of students know how the principle works and the basics of predicting a left or right shift, but students answers are often lacking in depth when they write out things.
It's not just about getting the correct equilibrium shift, but being able to justify why and linking this to other physical properties such as colours and solubility.
Here are some common mistakes students make when answering these questions:
- Not defining Le Chatelier's Principle in their answer
- Not fully justifying their choice on why the equilibrium shifted left or right
- Forgetting to discuss the changes in concentration caused by the shift in equilibrium
- Forgetting to link the shift to change in colour when the question has specified compounds have a certain colour
Each of these can cost you a mark on an area that if you get in an exam can often be easy guaranteed marks
Here is an Exemplar Answer to this question showing how your answer should be constructed:

Pay attention to:
- Le Chatelier's principle was defined
- The equilibrium shift was justified (and not just simply plainly written as shifting left or right --> you must say why)
- Link to reaction rate
- Comment on the effect on concentrations
- Linked to colours provided in the question
Bump!
As Year 11 comes to an end, many students in Chemistry may have underperformed and are looking to improve their skills prior to Year 12 or they ask the question about what do they need to know from Year 11 that is necessary for Year 12 content.
Here is a link to a post I made before that answers this question:
- What you need to know from Year 11 for Year 12 Chemistry
If you have any specific requests for concepts feel free to comment. I will be soon again adding tips and advice to this thread
As Year 11 comes to an end, many students in Chemistry may have underperformed and are looking to improve their skills prior to Year 12 or they ask the question about what do they need to know from Year 11 that is necessary for Year 12 content.
Here is a link to a post I made before that answers this question:
- What you need to know from Year 11 for Year 12 Chemistry
If you have any specific requests for concepts feel free to comment. I will be soon again adding tips and advice to this thread
Edzion education
Member
- Joined
- Aug 29, 2020
- Messages
- 38
- Gender
- Male
- HSC
- 2019
In order to unify your understanding for modules 5, 6 and 7 I typically teach my students how all the chemistry concepts link to the concept of Gibbs free energy. Understanding this makes chemistry an easier subject to grasp as well as improves your understanding hence your ability to answer questions for the HSC.
Regards,
Edzion Education
Regards,
Edzion Education
GanonRorf
New Member
- Joined
- Jun 1, 2021
- Messages
- 5
- Gender
- Female
- HSC
- 2021
My class spent a good 2-3 weeks on the process saponification, but my tutor said it's out of the syllabus? She said only the "structure and action" of soaps/ detergents, as well as environmental impacts, would be tested on. Is this true? I don't want to spend time studying something that won't be tested on. Thanks
Saponification as a process was required knowledge in the old syllabus as there was a specific syllabus dot point about knowing the method. The structure, action and uses is what the dot point states in the new syllabus. It's still a good idea though to have some knowledge about the process. You don't need to memorise like the full method probably but knowing the chemical equation for producing a soap or detergent would be to good to know as that could fall under the 'structure' aspect of that dot pointMy class spent a good 2-3 weeks on the process saponification, but my tutor said it's out of the syllabus? She said only the "structure and action" of soaps/ detergents, as well as environmental impacts, would be tested on. Is this true? I don't want to spend time studying something that won't be tested on. Thanks
