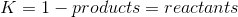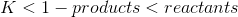Pretty sure Q isn't in the syllabus. Our teacher told us that the author of Conq Chem HSC was whacked when he wrote that section lol.
The way we were taught was that
Where a, b, c, d are the number of moles and A, B, C, D are the substances.
Then you put those in into this equation:
And the answer you get will mean:
Solids and liquid (not gas) water have a concentration of 1.
To answer your question, Q is calculated the same way that K is calculated, and is used in comparison to K. Q shows the point where the reaction is currently at, whereas K shows the point at which the reaction is at equilibrium. If that makes any sense at all. I don't even know if that's right tbh, it's the way I understood it when I read Conq Chem HSC. I'll read it again and edit this post accordingly.
"A reaction is at equilibrium if its reaction quotient, Q, is equal to the equilibrium constant, K. If the reaction is not at equilibrium, Q has a value different from K, and a chemical reaction occurs until Q equals K, that is, until equilibrium is reached."