-
Looking for HSC notes and resources? Check out our Notes & Resources page
Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
Oxidation and Reduction (Chem) (1 Viewer)
- Thread starter Two0Nine
- Start date
OILRIG: Oxidatoin Is Losing, Reduction is Gaining. Simple terms, if electron is on RHS, its an oxidation reaction, if its on LHS its a reduction.
ie}\rightarrow Cu^{2+}_{(aq)} + 2e^-) is an oxidation reactoin cos the copper is loosing of an electron. The reverse of this would be a reduction reaction
is an oxidation reactoin cos the copper is loosing of an electron. The reverse of this would be a reduction reaction
ie
Last edited:
B1andB2
oui oui baguette
In a displacement reaction, oxidation and reduction occur simultaneously. the more reactive metal (higher up on the data sheet) undergoes oxidation that is, it loses electrons, and the less reactive thing undergoes reduction, that is, it gains the electrons from the other thing. The reason why oxidation and reduction occur at the same time is because they can't just disappear, they need to go somewhere (i think it relates to the law of conservation of energy or something). To remember which one is loss and which one is gain, OIL RIG (oxidation is loss, reduction is gain!). When writing the half equations, the one that is oxidised, you reverse it from the data sheet (which is why there are two opposite arrows, refer to data sheet) and the one that undergoes reduction you just write it out as from the data sheet. Also these should form a Z.Can someone please explain everything about oxidation and reduction? (just a brief summary about it to wrap my head around it)
Also forgive my terrible technical words like things sorry, i learnt these at school recently so i'm a newbie at chem and could be wrong.
This is not entirely accurate, as indicatedOILRIG: Oxidatoin Is Losing, Reduction is Gaining. Simple terms, if electron is on RHS, its an oxidation reaction, if its on LHS its a reduction.
ieis an oxidation reactoin cos the copper is
gaining an electronan electron. The reverse of this would be a reduction reaction
Oxidation processes do have electrons on the RHS (and thus as products) as the process involve a loss of electrons. In the reaction quoted,
copper metal is being oxidised to copper(II) ions because the the copper atom is losing two electrons, and becoming positively charged in the process. In terms of oxidation states, the copper is changing from Cu0 to Cu+II, an increase in oxidation number, which is also a characteristic of any oxidation reaction.
Ah yeh, didn't realise what I was saying, even when I stated that oxidation is losing lol. Thanks for pointing it out!This is not entirely accurate, as indicated
Oxidation processes do have electrons on the RHS (and thus as products) as the process involve a loss of electrons. In the reaction quoted,
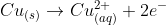
copper metal is being oxidised to copper(II) ions because the the copper atom is losing two electrons, and becoming positively charged in the process. In terms of oxidation states, the copper is changing from Cu0 to Cu+II, an increase in oxidation number, which is also a characteristic of any oxidation reaction.
