-
Looking for HSC notes and resources? Check out our Notes & Resources page
Make a Difference – Donate to Bored of Studies!
Students helping students, join us in improving Bored of Studies by donating and supporting future students!
What is colour of neutral in methyl orange? (1 Viewer)
- Thread starter enigma_1
- Start date
anomalousdecay
Premium Member
- Joined
- Jan 26, 2013
- Messages
- 5,757
- Gender
- Male
- HSC
- 2013
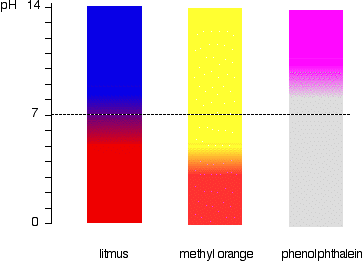
Going off this if the pH of solution is:
pH < 3.1, then Methyl Orange is Red.
3.1< pH < 4.4, then the Methyl Orange is Orange.
pH > 4.4 then the Methyl Orange is Yellow.
anomalousdecay
Premium Member
- Joined
- Jan 26, 2013
- Messages
- 5,757
- Gender
- Male
- HSC
- 2013
For some indicators like Methyl Orange, the colour change is slow as yellow and red are exhibited in the middle to produce orange.Yeah I'm still curious about how you thought 3.1 - 4.4 is yellow, and then also 4.4+ is yellow.
However, with something like phenolphthalein, there is a rapid change of colour from colourless to pink. However, from doing some titrations using this indicator, I found out that it still happens over quite a range, going from light pink to a very dark and vivid pink.
So generally, you must remember that with titrations, the colour change is gradual. You won't go instantly from red to yellow.
Or in the case of Bromothymol Blue for example, you won't go from blue straight to yellow instantly. Instead it will go from blue to green then to yellow (as a little blue and a little yellow together makes green).
