kawaiipotato
Well-Known Member
- Joined
- Apr 28, 2015
- Messages
- 463
- Gender
- Undisclosed
- HSC
- 2015
re: HSC Chemistry Marathon Archive
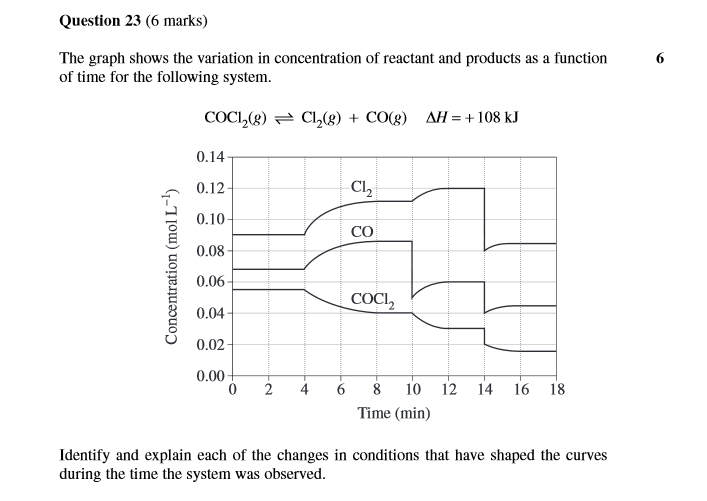
- From t = 0 to t = 4, the system is at equilibrium (constant molarities)
- At t = 4, the reaction was heated. By Le Chatelier's Principle, the system will shift towards the endothermic reaction, that is, the forward reaction. This causes the concentrations of reactants to decrease and the concentration of products to increase as the system attempts to return to equilibrium. (t = 4 to t = 10) shown by the curvature of the graph
- At t = 10, the sudden decrease in [CO] indicates that CO was removed from the system. This removal of CO will cause the equilibrium to shift (stated by LCP), right, further decreasing the concentration of reactants and increasing the concentration of products as it tries to return to equilibrium.
-At t = 14, as both [reactants] and [products] changed, this indicates a volume change and thus a pressure change. The increase in volume caused the [reactants] and [products] to decrease. By LCP, the equilibrium shifts towards the more gaseous moles reaction thus causing the graph to show an increase in [products] and a decrease in [reactants]
note: the shifting all happens according to the mole ratio which is 1:1:1
In dotpoints:ive been doing bio since last tuesday that i've forgotten some chemistry.
Need to sharpen up:
"
- From t = 0 to t = 4, the system is at equilibrium (constant molarities)
- At t = 4, the reaction was heated. By Le Chatelier's Principle, the system will shift towards the endothermic reaction, that is, the forward reaction. This causes the concentrations of reactants to decrease and the concentration of products to increase as the system attempts to return to equilibrium. (t = 4 to t = 10) shown by the curvature of the graph
- At t = 10, the sudden decrease in [CO] indicates that CO was removed from the system. This removal of CO will cause the equilibrium to shift (stated by LCP), right, further decreasing the concentration of reactants and increasing the concentration of products as it tries to return to equilibrium.
-At t = 14, as both [reactants] and [products] changed, this indicates a volume change and thus a pressure change. The increase in volume caused the [reactants] and [products] to decrease. By LCP, the equilibrium shifts towards the more gaseous moles reaction thus causing the graph to show an increase in [products] and a decrease in [reactants]
note: the shifting all happens according to the mole ratio which is 1:1:1
Last edited:


