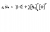-
Finished your HSC? Check out our University Discussion, Non-School forums or help out next year's HSC students! -
YOU can help the next generation of students in the community! Share your trial papers and notes on our Notes & Resources page
Lewis electron dot structures and ionic compounds (1 Viewer)
- Thread starter planino
- Start date
barbernator
Active Member
- Joined
- Sep 13, 2010
- Messages
- 1,439
- Gender
- Male
- HSC
- 2012
for sodium oxide, don't the sodium ions go on either side of the single oxygen ion?ok i will do them on paint now
actually, its probably best to split the Al up on the RHS, and draw both of them. People teach electron structures many different ways
barbernator
Active Member
- Joined
- Sep 13, 2010
- Messages
- 1,439
- Gender
- Male
- HSC
- 2012
yeh they do, i meant split the aluminium up that was on the right side of the equation. and yes put one either side.for sodium oxide, don't the sodium ions go on either side of the single oxygen ion?
Ah that clears it up, thanksyeh they do, i meant split the aluminium up that was on the right side of the equation. and yes put one either side.
nightweaver066
Well-Known Member
- Joined
- Jul 7, 2010
- Messages
- 1,585
- Gender
- Male
- HSC
- 2012
Seems right.
I don't think i've ever drawn anything as complicated as that back in year 11.
All i remember drawing are NaCl, HCl, water and simple diatomic molecules haha.
.....I knew that....To represent the equation you do
- 2Na. --> 2Na+ + 2electrons
- .O. + 2electrons --> O2-
By combining these two you get : 2 [Na-] + [ O2-] --> Na2O
Note the . represents an electron and + and 2- is the charge of the ion.
To show the ionic compound as a lewis structure you can use the drawing you posted
superSAIyan2
Member
- Joined
- Apr 18, 2012
- Messages
- 320
- Gender
- Male
- HSC
- 2013
I you did ngo that why did u post it a few deys ago.....I knew that....